Learn the steps of a preventive lifestyle to stop cognitive decline today.
The Scary Connection: Diabetes and Alzheimer’s (“Type 3”)
For decades, the medical community viewed the brain and the body’s metabolic system as distinct entities. We understood Type 1 diabetes as an autoimmune failure and Type 2 diabetes as a lifestyle-driven metabolic crisis of the blood. However, groundbreaking research has recently converged on a terrifying third classification: Type 3 Diabetes.
This term describes a specific form of brain insulin resistance that serves as a primary driver of cognitive decline and Alzheimer’s risk. The link is so profound that scientists now believe Alzheimer’s disease may actually be a late-stage metabolic disorder of the brain. Understanding this connection is no longer just a scientific curiosity—it is a critical necessity for anyone looking to preserve their memory and mental clarity as they age.
What is Type 3 Diabetes?
While not yet an official clinical diagnosis in all medical manuals, the term Type 3 Diabetes is used by researchers to describe the phenomenon where the brain loses its ability to respond to insulin.
In the rest of the body, insulin resistance leads to high blood sugar and weight gain. In the brain, insulin is responsible for far more than just "sugar processing." It is a master regulator of neurotransmitters, synaptic plasticity, and cellular repair. When the brain becomes "insulin resistant," neurons literally begin to starve for energy, leading to the rapid progression of memory loss and executive dysfunction.
The Science of Brain Insulin Resistance
To understand the "scary connection," we must look at how brain insulin resistance triggers a domino effect in our neural architecture.
- Glucose Metabolism Failure: The brain is the most energy-intensive organ in the body. If insulin signaling is impaired, neurons cannot effectively absorb glucose. Without fuel, they cannot communicate, and over time, they begin to wither and die.
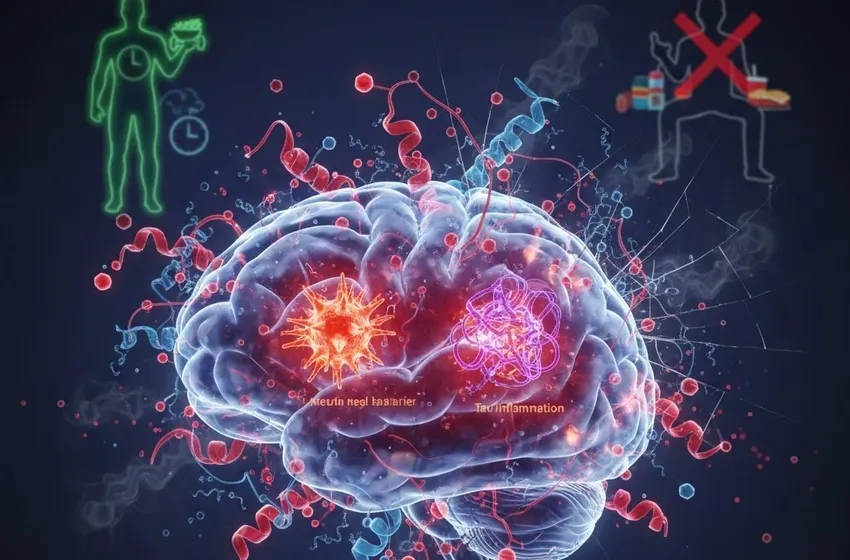
- The Amyloid-Beta Build-up: Research suggests that the enzyme responsible for breaking down insulin (Insulin-Degrading Enzyme) is also responsible for clearing out amyloid-beta plaques. When insulin levels are chronically high, the enzyme is too busy dealing with insulin to "clean" the brain, leading to plaque accumulation.
- Tau Hyperphosphorylation: Insulin resistance activates enzymes that cause Tau proteins to collapse into tangles, further choking the transport system within neurons.
The Silent Killer: Neuroinflammation
A major bridge between metabolic dysfunction and brain damage is neuroinflammation. When the brain struggles with high glucose and poor insulin signaling, it triggers an immune response.
Specialized cells called microglia, which are supposed to protect the brain, become chronically overactive. Instead of cleaning up debris, they begin secreting pro-inflammatory cytokines that damage healthy tissue. This chronic neuroinflammation acts like a slow-burning fire, destroying the blood-brain barrier and allowing systemic toxins to enter the central nervous system, further accelerating the transition from mild forgetfulness to full-blown dementia.
Analyzing the Alzheimer's Risk
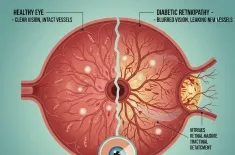
The statistics are sobering. Individuals with Type 2 diabetes have a 65% to 90% higher Alzheimer’s risk compared to those with healthy blood sugar levels. But the danger isn't limited to diagnosed diabetics.
Recent studies show that "pre-diabetic" levels of insulin resistance—often present for decades before a diagnosis—are enough to begin the process of shrinking the hippocampus, the brain’s memory center. This suggests that the seeds of Type 3 Diabetes are often sown in our 30s and 40s through sedentary behavior and high-sugar diets.
Reversing the Trend: A Preventive Lifestyle
The most empowering aspect of the research is that Type 3 Diabetes is largely a lifestyle-driven condition, which means it is also lifestyle-preventable. Adopting a preventive lifestyle is the most effective way to shield your brain from the ravages of insulin-related decay.
1. Metabolic Flexibility through Nutrition
To combat brain insulin resistance, the brain must become "metabolically flexible." Shifting away from refined sugars prevents insulin spikes, while incorporating healthy fats provides an alternative fuel source for neurons. The MIND Diet has been shown to slow cognitive aging significantly.
2. Physical Activity as Medicine
Exercise is a potent insulin sensitizer. Aerobic exercise increases the production of Brain-Derived Neurotrophic Factor (BDNF), which helps repair damaged neurons and improves the efficiency of insulin receptors.
3. Strategic Fasting and Sleep
Intermittent fasting allows insulin levels to drop enough to trigger "autophagy"—a cellular recycling process that clears out toxic proteins. Deep sleep is also essential, as it is when the brain flushes out metabolic waste.
Common Symptoms to Watch For
- Difficulty finding words or following conversations.
- Increased irritability or "mood crashes" after eating high-carb meals.
- Persistent "brain fog" that does not resolve with caffeine.
- Slowed processing speed (taking longer to complete familiar tasks).
| Feature | Type 2 Diabetes | Type 3 Diabetes (Alzheimer's Link) |
|---|---|---|
| Primary Location | Pancreas / Muscle / Liver | Central Nervous System (Brain) |
| Core Issue | Systemic Insulin Resistance | Brain Insulin Resistance |
| Key Symptom | High Blood Sugar / Fatigue | Cognitive Decline / Memory Loss |
The Future of Treatment
The recognition of Alzheimer’s as a metabolic disease has opened new doors for treatment. Clinical trials are currently investigating the use of intranasal insulin and GLP-1 agonists to see if they can halt or reverse the symptoms of dementia. However, addressing our metabolic health today remains the most effective strategy.
Final Thoughts
The connection between diabetes and Alzheimer's is a wake-up call. Our brain health is inextricably linked to how we fuel our bodies. By understanding the mechanics of Type 3 Diabetes, we can move toward a proactive strategy of prevention. Protecting your insulin sensitivity is the single best investment you can make for your future self.