Explore how in vivo editing and base editing using CRISPR-Cas9 are revolutionizing single-gene correction
Advances in CRISPR for Genetic Disease: The Frontier of Precision Medicine
The landscape of modern medicine is undergoing a seismic shift, driven by the rapid evolution of the CRISPR-Cas9 system. Once a humble bacterial defense mechanism, it has transformed into the most powerful toolkit in biotechnology. As we move deeper into 2025, the focus has shifted from merely understanding how to "cut" DNA to mastering how to "rewrite" it with surgical precision. The most significant breakthroughs are occurring in in vivo editing and base editing, which are opening new doors for the treatment of genetic disorders that were once considered incurable.
This article explores the latest technical leaps in gene editing, focusing on how these tools provide single-gene correction and their expanding therapeutic applications in clinical settings.
Understanding the Core: CRISPR-Cas9 Evolution
At its simplest, CRISPR-Cas9 acts as a pair of molecular scissors. Guided by a small piece of RNA (gRNA), the Cas9 enzyme identifies a specific sequence in the genome and creates a double-strand break. While this is effective for "knocking out" harmful genes, the cell’s natural repair process—Non-Homologous End Joining (NHEJ)—is often prone to errors, leading to random insertions or deletions (indels).
For single-gene correction, researchers are now moving beyond these "blunt cuts." By leveraging Homology-Directed Repair (HDR) and newer, more refined Cas variants, scientists can now swap out a mutated sequence for a healthy one. This precision is vital for treating conditions where a single "typo" in the DNA code results in a devastating disease.
The Rise of In Vivo Editing: Treating Disease Inside the Body
One of the most significant hurdles in genetic medicine has been delivery. Historically, many treatments were ex vivo, meaning cells were removed from the patient, edited in a lab, and then infused back (as seen in recent FDA-approved therapies for sickle cell disease). However, the future lies in in vivo editing—delivering the CRISPR-Cas9 machinery directly into the patient’s body.
Breakthroughs in Delivery Systems
The success of in vivo editing depends entirely on reaching the target organ without being neutralized by the immune system. Recent advances include:
-
Lipid Nanoparticles (LNPs): These fatty spheres encapsulate the CRISPR components, allowing them to travel through the bloodstream. This has been particularly successful in targeting the liver to treat conditions like transthyretin amyloidosis.
-
Viral Vectors (AAV): Engineered Adeno-Associated Viruses are used to "infect" specific tissues—like the retina or muscle—with the genetic instructions for the CRISPR system.
-
Tissue-Specific Targeting: In 2025, new research has demonstrated the ability to program delivery vehicles to bypass the liver and head straight for the heart or central nervous system, broadening the scope of therapeutic applications.
Base Editing: The "Pencil" of Genetic Engineering
If traditional CRISPR-Cas9 is a pair of scissors, base editing is a pencil. Developed to address the risks associated with double-strand DNA breaks, base editors allow for the direct, permanent conversion of one DNA base into another without cutting the DNA backbone.
How It Works
Base editors use a "deactivated" or "nicked" version of the Cas9 protein (dCas9 or nCas9). Instead of cutting, it carries a deaminase enzyme to the target site.
-
Cytosine Base Editors (CBEs): Convert a C•G base pair into a T•A base pair.
-
Adenine Base Editors (ABEs): Convert an A•T base pair into a G•C base pair.
This technology is revolutionary for single-gene correction. Since approximately 50% of human genetic disorders are caused by "point mutations" (a single base pair change), base editing offers a safer, more predictable way to "correct the typo" without the risk of unintended chromosomal rearrangements.
Single-Gene Correction in Rare and Common Diseases
The clinical impact of these advances is most visible in the treatment of single-gene disorders. By targeting the root cause of the disease at the molecular level, we are seeing "one-and-done" cures enter the horizon.
| Disease | Mutation Type | CRISPR Approach | Status (2025) |
| Sickle Cell Disease | Point Mutation | Ex Vivo / In Vivo | FDA Approved / Trials |
| Leber Congenital Amaurosis | Single-Gene Mutation | In Vivo (Retinal) | Clinical Trials |
| Hereditary Tyrosinemia | Single-Gene Mutation | Base Editing | Pre-clinical / Early Trials |
| Familial Hypercholesterolemia | Point Mutation | In Vivo (Liver) | Active Clinical Trials |
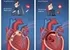
Case Study: Progeria and Base Editing
Hutchinson-Gilford progeria syndrome is a rare, fatal genetic condition characterized by rapid aging. It is caused by a single C-to-T mutation. Recent studies using base editing in mouse models have shown the ability to correct this mutation, significantly extending lifespan and improving cardiovascular health. This serves as a primary example of how gene editing can tackle the most complex genetic disorders by fixing a single letter of DNA.
The Future of Therapeutic Applications
As we look toward the late 2020s, the therapeutic applications of CRISPR are expanding beyond rare diseases into chronic, widespread conditions.
-
Cardiovascular Health: Scientists are using in vivo editing to "knock out" genes in the liver that produce LDL (bad) cholesterol. This could potentially replace daily statins with a single injection.
-
Infectious Disease: CRISPR is being tested to "hunt" and destroy the latent DNA of viruses like HIV and Hepatitis B within the human body.
-
Neurodegenerative Diseases: New CRISPR platforms are being designed to cross the blood-brain barrier to target the genetic drivers of Alzheimer's and Huntington's disease.
The journey from a laboratory curiosity to a clinical reality has been remarkably short. As gene editing continues to refine its precision through base editing and its reach through in vivo editing, the dream of curing genetic disorders with a single treatment is no longer science fiction—it is the new standard of medicine.