Discover how liquid biopsy and circulating tumor DNA (ctDNA) use simple blood tests for early cancer detection, screening, and recurrence monitoring
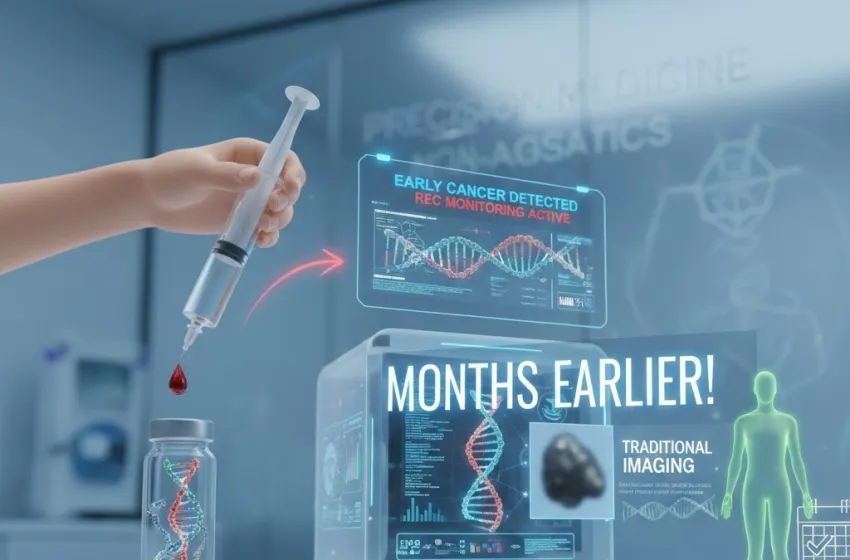
The landscape of cancer diagnosis and surveillance is undergoing a profound transformation, spearheaded by the advent of the liquid biopsy. This groundbreaking technology is shifting the paradigm from invasive procedures to simple, non-invasive blood tests that promise to detect cancer far earlier than traditional methods, and enable precise, real-time tracking of disease status.
At its core, the promise of the liquid biopsy lies in its ability to analyze minute, tell-tale fragments of genetic material shed by cancer cells into the bloodstream—a key component known as circulating tumor DNA (ctDNA). This scientific marvel is paving the way for revolutionary advancements in early cancer detection, personalized medicine, and long-term recurrence monitoring.
The Science of the Liquid Biopsy and ctDNA
What is a Liquid Biopsy?
A biopsy has historically involved surgically removing a small piece of suspicious tissue (a solid biopsy) for microscopic and molecular analysis. While indispensable, this procedure is invasive, often painful, carries risks, and only captures a snapshot of the tumor at one specific site and time.
The liquid biopsy, in contrast, is a minimally invasive technique that analyzes cancer-related biomarkers—such as circulating tumor DNA (ctDNA), circulating tumor cells (CTCs), and exosomes—found in body fluids, most commonly blood plasma. The simplicity of collecting a sample via a routine venipuncture (a simple blood draw) makes this technique highly repeatable and far more patient-friendly.
Understanding Circulating Tumor DNA (ctDNA)
The key biomarker driving the utility of the liquid biopsy is circulating tumor DNA (ctDNA). Tumors, like all tissues, are constantly shedding cells. As cancer cells die (through apoptosis or necrosis) or are actively secreted, they release their fragmented DNA into the bloodstream. This DNA, which harbors the specific genetic mutations and epigenetic alterations characteristic of the tumor, is what scientists analyze.
It’s crucial to differentiate ctDNA from the vast majority of non-tumor DNA fragments in the blood, which come from normal, healthy cells (collectively known as cell-free DNA or cfDNA). The challenge, and the triumph, of liquid biopsy technology is its ultra-high sensitivity—the ability to find and analyze the tiny fractions of ctDNA that make up as little as 0.01% of the total cfDNA, especially in the early stages of cancer.
The Mechanism: Simple Blood Tests for Complex Genetics
The process begins with a standard blood test. Once the blood sample is collected, the plasma (the liquid component) is separated. Next, cutting-edge molecular techniques are employed to isolate and analyze the cfDNA, specifically searching for ctDNA.
Modern methods, such as Digital Polymerase Chain Reaction (dPCR) and Next-Generation Sequencing (NGS), have the requisite sensitivity to detect the minute quantities of mutated DNA. They look for tumor-specific characteristics—like somatic mutations, aberrant methylation patterns, or chromosomal rearrangements—that confirm the presence and nature of the cancer. Using simple blood tests to find circulating tumor DNA (ctDNA) for screening and monitoring cancer recurrence long before traditional imaging is the core functional advantage.
Applications in Early Cancer Detection
The potential for liquid biopsy in early cancer detection and cancer screening is arguably its most transformative application.
Multi-Cancer Early Detection (MCED) Screening
Traditional cancer screening is often organ-specific (e.g., mammography for breast cancer, colonoscopy for colorectal cancer). However, a single liquid biopsy blood test can simultaneously screen for the presence of multiple cancer types (Multi-Cancer Early Detection or MCED). This is done by casting a wide net for various cancer-specific genetic and epigenetic markers in the ctDNA.
- Earlier Diagnosis: By detecting ctDNA signals, which appear as the tumor begins to shed DNA, liquid biopsies can flag cancer long before a tumor is large enough to cause symptoms or be visible on an imaging test (like a CT scan or ultrasound).
- Improved Outcomes: Detecting cancer in its earliest stages (Stage I or II) is statistically linked to significantly better patient outcomes and higher survival rates. The hope is to make a routine annual blood test a comprehensive form of cancer screening that significantly increases the rate of early-stage diagnoses.
Screening for High-Risk Individuals
Liquid biopsy is particularly valuable for individuals at high risk due to family history or known genetic predispositions. Regular blood tests can provide frequent, non-invasive surveillance, offering peace of mind or, crucially, the earliest possible warning sign.
Recurrence Monitoring: The Guardian Against Relapse
Beyond initial detection, liquid biopsy is rapidly becoming an indispensable tool for recurrence monitoring after primary treatment (surgery, chemotherapy, or radiation).
Detecting Minimal Residual Disease (MRD)
After a tumor is removed, there's always a risk that a tiny number of cancer cells—known as Minimal Residual Disease (MRD)—remain in the body, undetected by conventional means. This MRD is the source of future relapse.
Liquid biopsy can monitor for the presence of ctDNA after surgery. If the tumor-specific ctDNA is undetectable, it suggests successful eradication of the cancer. If, however, the ctDNA reappears—even at incredibly low levels—it signals impending relapse, often many months before any physical symptoms or radiographic evidence is detectable.
Intervening Long Before Traditional Imaging
This capability allows clinicians to intervene with further treatment long before traditional imaging can confirm the disease has returned. The goal is pre-emptive treatment when the tumor burden is minimal, making therapy significantly more effective. This is a critical advantage over the traditional wait-and-see approach, where clinicians wait for the cancer to become symptomatic or visible on a scan before initiating second-line therapy.
The ability to use routine, non-invasive blood tests for such sensitive recurrence monitoring transforms post-treatment surveillance from an infrequent, anxiety-provoking event to a proactive, real-time assessment of disease status.
ctDNA in Personalized Treatment and Monitoring
The utility of ctDNA extends deep into personalized cancer management for patients who are actively undergoing treatment.
Guiding Targeted Therapies
CtDNA analysis can identify the specific genetic mutations driving a patient’s cancer. This information allows oncologists to select targeted therapies—drugs designed to block the activity of a specific mutated protein—offering a more personalized and potentially more effective treatment plan.
Real-Time Treatment Response Assessment
By performing sequential liquid biopsy blood tests, clinicians can track how the ctDNA levels change during therapy:
- Decreasing ctDNA: Signals the treatment is working and the tumor is shrinking.
- Stable or Increasing ctDNA: Suggests the treatment is ineffective, prompting an immediate need to switch therapies without waiting for physical or radiological changes.
- Emergence of New Mutations: ctDNA can reveal new mutations that confer drug resistance. This allows doctors to anticipate treatment failure and switch to an alternative regimen before the existing one completely loses efficacy.
This dynamic, real-time recurrence monitoring and therapy guidance overcomes the limitations of infrequent solid biopsies and delayed imaging results, enabling true precision oncology.
Challenges and Future Directions
While the promise of the liquid biopsy is immense, challenges remain for its widespread clinical adoption:
- Sensitivity and Specificity: In the general population, the concentration of ctDNA in very early-stage cancer can be incredibly low, making false-negative results a risk. Continual advances in assay sensitivity are crucial.
- Origin of Signal: When a positive ctDNA signal is detected during cancer screening in an asymptomatic individual, determining the location of the cancer (the tissue of origin) is essential for follow-up and treatment. Ongoing research is focused on using methylation patterns in the ctDNA to accurately pinpoint the cancer type.
- Standardization: As a new technology, standardizing sample processing, analysis protocols, and result interpretation across different institutions is necessary.
The future of the liquid biopsy will involve its integration with other diagnostic tools, leveraging Artificial Intelligence (AI) and Machine Learning to better analyze complex ctDNA data, and expanding its application from high-risk monitoring to broad population-based cancer screening. This non-invasive technology is not just an incremental improvement; it is a fundamental shift in how cancer is detected, tracked, and treated, moving us closer to a future where cancer is routinely caught and cured at its most treatable stage.
Conclusion
The liquid biopsy, driven by the detection of circulating tumor DNA (ctDNA) via simple blood tests, represents one of the most exciting breakthroughs in modern oncology. Its advantages over traditional, invasive procedures—offering real-time, comprehensive, and non-invasive assessment—make it an unparalleled tool for both early cancer detection and precise recurrence monitoring. By identifying cancer's molecular fingerprint in the blood long before traditional imaging can, this technology is poised to dramatically improve survival rates and usher in a new era of proactive and personalized cancer care.