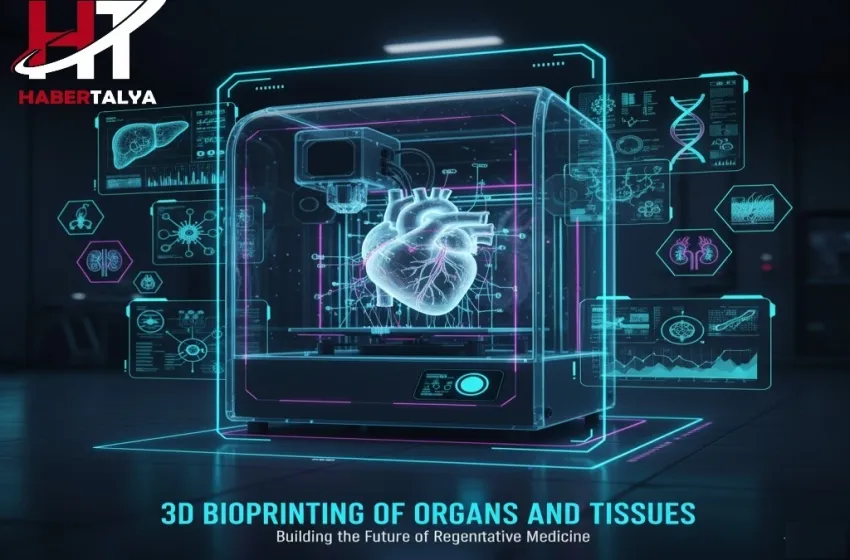
3D bioprinting represents a monumental leap in the fields of regenerative medicine and tissue engineering. Moving beyond the fabrication of inert structures, this technology uses biological materials—cells and growth factors—to create living structures layer by layer.
3D bioprinting represents a monumental leap in the fields of regenerative medicine and tissue engineering. Moving beyond the fabrication of inert structures, this technology uses biological materials—cells and growth factors—to create living structures layer by layer. The ultimate ambition is to solve the global crisis in organ transplant by providing personalized, viable organs on demand.
This article explores the transformative potential of this technology, specifically detailing the current status of printing functional tissues (e.g., skin, cartilage) and the long-term goal of printable organs for transplant. We delve into the intricacies of bio-ink development, the challenges of vascularization, and the profound ethical considerations that accompany the ability to manufacture life.
Defining 3D Bioprinting and Bio-Ink
3D bioprinting is an additive manufacturing process that combines cells, scaffolding materials, and precise deposition techniques to construct three-dimensional, living structures that mimic natural tissues. Unlike conventional 3D printing which uses polymers or metals, bioprinting relies on bio-ink.
The Critical Role of Bio-Ink
The bio-ink is the cornerstone of successful bioprinting. It is a viscoelastic hydrogel material containing living cells, growth factors, and other biomolecules necessary to support cell survival, proliferation, and differentiation once printed. The design of the bio-ink must satisfy three critical criteria simultaneously:
- Printability (Rheological Properties): It must have the correct viscosity to be extruded accurately without clogging the nozzle and maintain its printed shape (structural fidelity) post-printing.
- Biocompatibility: The material must be non-toxic and provide an environment that encourages cell viability and function.
- Mechanical Stability: The resulting printed structure must possess sufficient strength and stiffness to withstand handling and physiological forces.
Common materials used in bio-inks include natural polymers (alginate, collagen, gelatin, fibrin) and synthetic polymers (Pluronic, PEG).
Current Status: Printing Functional Tissues
The goal of tissue engineering is to create functional tissues that can replace, repair, or enhance biological function. 3D bioprinting has already achieved significant milestones in printing simpler, avascular (lacking blood vessels) structures.
Successes in Avascular Tissues
The current status of printing functional tissues is most advanced in structures that do not heavily rely on a complex network of blood vessels for nutrient supply:
- Skin: Bioprinted skin models are routinely used in pharmaceutical testing, toxicology studies, and burn treatments. These models often successfully integrate dermal (fibroblasts) and epidermal (keratinocytes) layers, providing a functional barrier. The goal is moving toward full-thickness, personalized skin grafts for patients, minimizing rejection risks.
- Cartilage and Bone: Cartilage is naturally avascular, making it an excellent candidate. Bioprinted cartilage constructs have been successfully engineered using chondrocytes (cartilage cells) within hydrogels. Similarly, bone scaffolds have been printed, often using porous materials seeded with osteoblasts, to promote bone regeneration in orthopedic surgery.
- Corneal Tissue: Researchers have successfully printed corneal stroma cells (keratocytes) in a collagen-based bio-ink, paving the way for eventual lab-grown transplants to treat common vision problems.
These functional tissues are currently serving as vital platforms for in vitro drug screening and disease modeling, reducing reliance on animal testing and accelerating therapeutic discovery.
The Long-Term Goal: Printable Organs for Transplant
While printing simple tissues is a reality, the long-term goal of manufacturing entire, complex organs for organ transplant remains one of the greatest technical challenges in regenerative medicine. The need is immense: thousands of people die annually waiting for a suitable donor organ (kidney, heart, liver).
The Challenge of Vascularization
The primary barrier to printing large, complex organs is vascularization—the creation of an intrinsic network of blood vessels (arteries, veins, and capillaries) necessary to supply oxygen and nutrients to every cell in the structure and remove waste. Without vascularization, cells more than 100-200 micrometers away from a nutrient source will rapidly die.
- Sacrificial Inks: One key strategy involves using "sacrificial bio-inks" (e.g., pluronic F-127) that are printed to form temporary channels within the organ scaffold. Once the primary structural bio-ink is cured, the sacrificial ink is melted and flushed out, leaving behind a network of hollow microchannels that can be lined with endothelial cells (vessel cells).
- Organ-on-a-Chip: While not a transplant solution, "organ-on-a-chip" models—small, functional, vascularized micro-tissues—are being printed to simulate complex organ function (e.g., liver or kidney) for drug testing, representing a crucial intermediate step in achieving vascular complexity.
The Challenge of Functionality and Maturation
Even if an organ structure can be printed and successfully perfused, it must mature into a fully functional tissue. A printed heart must beat rhythmically, and a printed liver must perform complex metabolic and detoxification processes. This requires precise control over cell differentiation, tissue remodeling, and electrical or fluidic coupling between different cell types, a process that can take weeks or months in a bioreactor environment.
By integrating these keywords, the content comprehensively covers the technology's application from the fundamental material (the bio-ink) to the aspirational clinical outcome (a fully functional tissues for organ transplant).
Ethical and Regulatory Considerations
The ability to create life-like structures raises profound ethical considerations that span patient safety, access, and the fundamental nature of manufactured biology.
1. Patient Safety and Clinical Translation
Before printed organs can move from the lab to the operating room, safety, and long-term viability must be proven. The risk of tumor formation from progenitor or stem cell use, the long-term stability of the bio-ink scaffold in vivo, and the potential for device failure are paramount concerns. Regulatory bodies must establish new approval pathways for these complex, living medical products, which combine devices, cells, and drugs.
2. Equity and Access
If 3D bioprinting successfully solves the organ transplant crisis, the technology must be accessible to all, not just the wealthy. The high cost of initial research and the specialized infrastructure required for patient-specific printing could exacerbate existing healthcare disparities, potentially leading to a two-tiered system for life-saving organs.
3. Bioprinting Human Enhancement
The ethical boundary between therapeutic use (replacing a diseased kidney) and enhancement (printing stronger, superior functional tissues) is already a topic of debate. The potential misuse of 3D bioprinting for non-medical or military applications requires clear ethical guidelines and international governance frameworks.
Conclusion: Bridging the Gap
3D bioprinting has firmly established itself as a foundational technology in regenerative medicine. While we have successfully crossed the first threshold—printing simple, avascular functional tissues like skin and cartilage for research and limited therapeutic use—the journey to providing complete, vascularized printable organs for transplant is ongoing.
Success hinges on overcoming the vascularization hurdle, perfecting the bio-ink materials, and mastering the long-term maturation process within advanced bioreactors. As researchers bridge the gap between structural complexity and genuine biological function, 3D bioprinting stands poised to not only revolutionize tissue engineering but fundamentally redefine healthcare, eliminating the waiting list and the specter of immune rejection forever.
Frequently Asked Questions (FAQ)
What is the fundamental difference between 3D bioprinting and conventional 3D printing?
Conventional 3D printing uses inert materials (plastics, metals) to build non-living objects. 3D bioprinting uses living materials, primarily bio-ink (a mixture of hydrogels and living cells), to create biological structures and functional tissues for regenerative medicine.
Why is vascularization the main challenge in printing full organs for organ transplant?
Vascularization (creating a network of blood vessels) is essential because cells in large organs need oxygen and nutrients. Without a blood supply, cells more than 100-200 micrometers from a nutrient source will die rapidly, preventing the creation of large, functional tissues like a liver or kidney.
What types of functional tissues have been successfully printed for immediate use?
The most successful printed functional tissues are avascular or simple structures, including multi-layered skin models (used for drug testing), cartilage structures, and small liver or heart organ-on-a-chip models used for studying disease.
What are the key properties that a good bio-ink must possess?
A good bio-ink must have three key properties: Printability (to hold shape after extrusion), Biocompatibility (to keep the cells alive and healthy), and Mechanical Stability (to support the structure's shape).
What is the primary ethical consideration facing the widespread adoption of 3D bioprinting?
The primary ethical consideration is equity and access. If 3D bioprinting provides life-saving printable organs, there is a significant risk that the high cost of personalized technology could create severe disparities, limiting access to only the wealthy and exacerbating health inequalities.
How is the problem of vascularization being addressed in 3D bioprinting research?
The problem is being addressed using sacrificial bio-inks. These materials are printed to create a temporary network of channels within the tissue. They are then liquefied and flushed out, leaving behind a hollow vascular network that can be lined with endothelial cells to create perfusable, functional tissues.
What are the advantages of using 3D bioprinting for drug testing instead of traditional methods?
3D bioprinting allows researchers to create highly complex, patient-specific functional tissues (like "organ-on-a-chip" models) that mimic human disease much more accurately than traditional 2D cultures or animal models. This improves the predictability of drug efficacy and toxicology, accelerating regenerative medicine research.
Explain how 3D bioprinting could potentially solve the immune rejection issue in organ transplant.
3D bioprinting aims to solve rejection by using a patient's own cells (autologous cells) to print the replacement organ. Since the organ is derived from the patient's genetic material, the body's immune system should recognize it as "self," eliminating the need for long-term immunosuppressive drugs.
What is the difference between tissue engineering and 3D bioprinting?
Tissue engineering is the broader field focused on creating functional tissues to repair or replace damaged parts. 3D bioprinting is a specific, high-precision technique (an additive manufacturing method) used within tissue engineering to rapidly and accurately arrange cells and biomaterials into complex structures.
What is the significance of printing avascular structures like cartilage first?
Cartilage is naturally avascular, meaning it does not contain blood vessels. This makes it an ideal starting point for 3D bioprinting because it bypasses the most complex technical hurdle—vascularization—allowing researchers to focus on optimizing cell survival, bio-ink formulation, and mechanical stability.