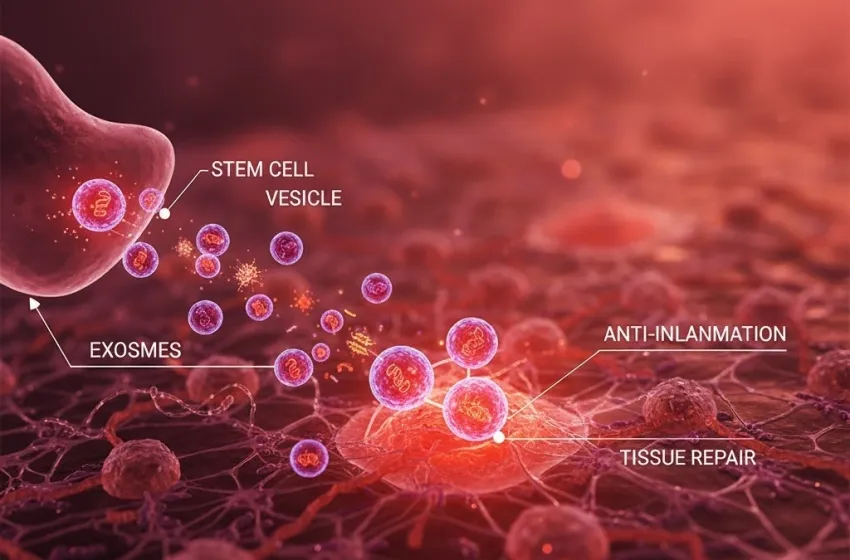
Learn how stem cell vesicles deliver therapeutic cargo for potent tissue repair and anti-inflammation.
Exosome therapy has emerged as a revolutionary, cell-free frontier in regenerative medicine. This innovative approach leverages nature's own communication system—tiny, membrane-bound sacs known as exosomes, which are a specific type of extracellular vesicle (EV). These nanoparticles, typically ranging from 30 to 150 nanometers, are secreted by virtually all cell types, but those derived from mesenchymal stem cells (MSCs) are of particular interest due to their potent therapeutic cargo and strong regenerative capabilities.
The Mechanism of Cellular Communication
The core concept of exosome therapy is the use of tiny vesicles (exosomes) released by stem cells to deliver therapeutic cargo for tissue repair and anti-inflammation.
Rather than the whole stem cell vesicles physically integrating into damaged tissue—which carries risks of immune rejection and unwanted cell differentiation—exosomes act as targeted messengers. They are shuttled from a parent cell (like an MSC) and travel through body fluids until they reach recipient cells at the site of injury or inflammation.
- Targeted Delivery: Exosomes possess surface proteins that allow them to selectively bind to damaged or inflamed cells, offering a highly specific form of treatment.
- Cargo Delivery: Once at the target cell, the exosome can fuse with the cell membrane or be absorbed via endocytosis, releasing its therapeutic cargo directly into the recipient cell's cytoplasm.
This delivered cargo then works at the molecular level to "re-program" the damaged cells.
The Therapeutic Cargo and Its Regenerative Power
The contents of the exosomes, which reflect the health and function of the parent cell, are the keys to their therapeutic effect. This complex therapeutic cargo is what drives tissue repair and anti-inflammation:
| Component | Function in Tissue Repair |
|---|---|
| Proteins & Growth Factors | Directly stimulate cell proliferation, migration, and differentiation (e.g., promoting new blood vessel formation, known as angiogenesis). |
| mRNA & MicroRNA (miRNA) | Function as genetic instructions. When delivered, they can alter the gene expression of the recipient cell, shifting its phenotype toward a reparative state and away from an inflammatory or apoptotic (cell death) state. |
| Lipids | Contribute to membrane integrity and participate in intracellular signaling pathways that promote healing. |
Through the transfer of this cargo, exosomes orchestrate a systemic regenerative response, which includes:
- Tissue Repair and Regeneration: Stimulating native progenitor cells and promoting the creation of structural proteins like collagen and elastin, critical for healing musculoskeletal injuries, bone, cartilage, and skin wounds.
- Anti-Inflammation: Modulating the immune response by shifting immune cells (like macrophages) from a pro-inflammatory (M1) phenotype to an anti-inflammation and pro-regenerative (M2) phenotype, which helps to resolve chronic inflammation that often impedes healing.
Advantages in Regenerative Medicine
The transition from whole-cell therapies to exosome therapy represents a significant step forward in regenerative medicine. As a cell-free therapeutic agent, exosomes offer several key advantages:
- Lower Immunogenicity: They are less likely to trigger a strong immune response or be rejected compared to whole stem cells.
- Stability and Storage: They can be isolated, characterized, stored, and sterilized more easily than living cells, simplifying logistical and regulatory pathways.
- Ability to Cross Barriers: Their nanoscale size allows them to potentially traverse biological barriers, such as the blood-brain barrier, opening up new avenues for treating neurodegenerative disorders.
While research is ongoing and clinical trials are necessary to achieve widespread regulatory approval, the potent, targeted, and cell-free nature of exosomes solidifies their position as a leading candidate for the next generation of therapeutics.