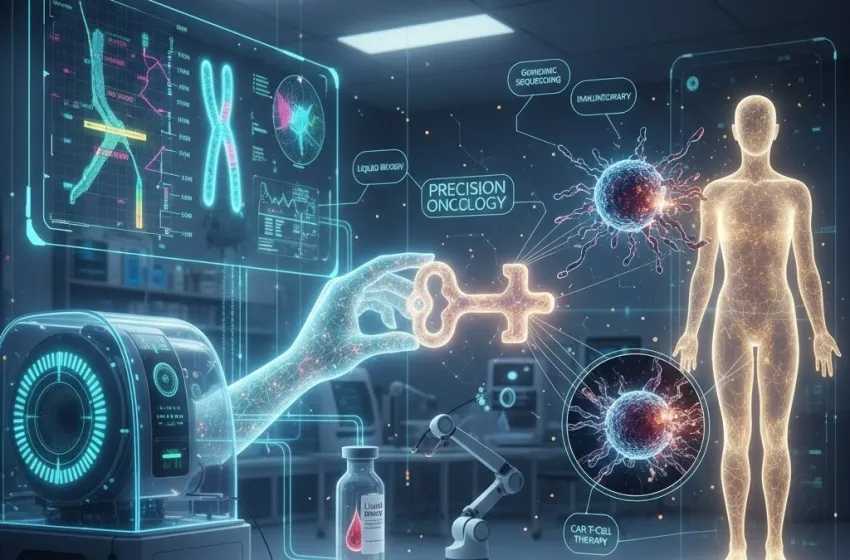
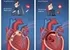
Unlock Precision Oncology via genomic sequencing, tumor profiling, liquid biopsy, CAR T-cell therapy, and personalized cancer vaccines for better outcomes.
Cancer, for decades, was largely viewed and treated as a collection of diseases defined by the organ of origin—lung cancer, breast cancer, colon cancer. While this classification was foundational, it led to a "one-size-fits-all" approach, where patients with the same cancer type received identical, often highly toxic, treatments like traditional chemotherapy. This paradigm is now being fundamentally revolutionized by Precision Oncology, a revolutionary discipline that views cancer not by its location but by its unique and underlying genetic drivers. This new era of cancer care is defined by Tailoring cancer treatment based on the unique genetic signature of a patient's tumor for more effective and less toxic outcomes. By integrating the power of genomic sequencing with the strategic force of immunotherapy, we are moving from generalized toxicity to targeted cure.
The Foundational Pillar: Genomic Sequencing and Tumor Profiling
The journey toward personalized cancer treatment begins with understanding the enemy at its most granular level: the tumor profiling of its DNA and RNA. Every cancer arises from a unique accumulation of somatic (acquired) mutations, which differ vastly from person to person, even among those with the same diagnosis.
Mapping the Genetic Landscape
The cornerstone of this process is high-throughput genomic sequencing, which analyzes the entire or relevant portions of the tumor's genetic code. This deep analysis reveals the specific mutations, gene amplifications, deletions, and fusions that are driving uncontrolled cell growth. The goal is to identify actionable mutations—genetic alterations for which a targeted drug or a specific immunotherapy approach is known or suspected to be effective.
- Next-Generation Sequencing (NGS): NGS technologies allow for the rapid and simultaneous sequencing of hundreds of genes, whole exomes, or even entire genomes from a single tumor sample. This provides a comprehensive map of the tumor’s genetic vulnerabilities.
- The Neoantigen Blueprint: Crucially, genomic sequencing can identify neoantigens—novel proteins that arise from these unique tumor mutations. These neoantigens are foreign to the patient’s body and represent the perfect targets for the immune system, acting as the 'most wanted' posters for immune cells.
The Rise of Liquid Biopsy
While traditional tumor profiling requires an invasive surgical procedure to obtain a tissue sample, a breakthrough technology known as liquid biopsy is transforming the diagnostic pipeline. This simple blood test detects circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) released by the tumor into the bloodstream.
- Non-Invasive Monitoring: Liquid biopsy allows for repeated, non-invasive monitoring of a tumor's evolution in real-time. This is critical because tumors are dynamic; they can develop new mutations—and thus drug resistance—while a patient is on treatment.
- Early Detection and Resistance Tracking: It can be used for earlier detection of recurrence and, most importantly, for tracking the emergence of resistance mutations, allowing clinicians to switch therapies before the patient experiences clinical relapse. This ability to adapt treatment rapidly embodies the core principle of Precision Oncology.
Immunotherapy: Harnessing the Body's Own Arsenal
Once the tumor's genetic signature is mapped, the next step is to select the therapeutic strategy. While targeted small-molecule drugs are part of the arsenal, the most transformative advancements have come from immunotherapy, which leverages and enhances the patient's own immune system to fight the cancer.
The Personalized Cancer Vaccines Revolution
The identification of unique neoantigens through genomic sequencing has directly enabled the creation of personalized cancer vaccines. Unlike traditional vaccines that prevent infectious diseases, these therapeutic vaccines are designed to treat an existing cancer by teaching the immune system to recognize the tumor's specific neoantigens.
- Tumor Analysis: The patient’s tumor is sequenced to identify its unique somatic mutations.
- Neoantigen Prediction: Sophisticated computational algorithms predict which of these mutations will produce a highly immunogenic neoantigen.
- Vaccine Manufacture: A vaccine containing the most promising neoantigens (often as synthetic peptides or mRNA) is custom-manufactured.
- Immune Activation: The vaccine is administered, activating cytotoxic T cells to seek out and destroy only the cells displaying those specific neoantigens—the cancer cells.
This highly targeted approach aims to generate a long-term, memory-driven immune response, preventing recurrence with minimal damage to healthy tissues.
CAR T-Cell Therapy: Living Drugs
Another monumental leap in personalized immunotherapy is CAR T-cell therapy (Chimeric Antigen Receptor T-cell therapy). This process involves genetically modifying a patient's own T-cells to transform them into "living drugs" engineered to hunt down and kill cancer cells expressing a specific surface marker (antigen).
- Harvest and Engineering: T-cells are harvested from the patient's blood (apheresis). In a lab, a Chimeric Antigen Receptor (CAR) gene is introduced to reprogram the T-cells.
- Targeted Destruction: The resulting CAR T-cells are expanded into millions and then infused back into the patient, where they act as guided missiles, binding to the tumor antigen and initiating a potent and lasting immune response.
- Current Success: CAR T-cell therapy has achieved remarkable success in treating certain hematological malignancies (blood cancers) that are resistant to conventional therapies, offering curative potential to patients who previously had few options. The ongoing challenge is adapting this powerful approach to solid tumors, where the tumor microenvironment presents significant barriers.
The Clinical Integration: From Data to Decision
The true power of Precision Oncology lies in the seamless integration of these diagnostic and therapeutic technologies. The decision-making process for a patient's treatment is no longer based on population averages but on their unique genetic reality.
The Molecular Tumor Board
In many leading cancer centers, patient cases are reviewed by a Molecular Tumor Board (MTB)—a multidisciplinary team of oncologists, molecular pathologists, bioinformaticians, and genetic counselors. This board interprets the complex data generated by genomic sequencing and tumor profiling to recommend the most precise course of action, which could range from a targeted small molecule inhibitor to enrollment in a trial for personalized cancer vaccines or CAR T-cell therapy.
Measuring Effectiveness and Safety
The promise of personalized treatment is twofold: more effective therapy and less toxic outcomes.
- Enhanced Efficacy: By targeting the precise molecular vulnerability that drives a tumor, targeted therapies often result in higher initial response rates compared to untargeted, broad-spectrum chemotherapy. Furthermore, immunotherapy can induce durable, long-term responses that transform a chronic disease into a manageable one, or even a cure.
- Reduced Toxicity: Traditional chemotherapy kills rapidly dividing cells indiscriminately, leading to severe side effects like hair loss, nausea, and immune suppression. In contrast, targeted drugs and immunotherapies are designed to interact specifically with cancer-related molecules or cells. While immunotherapies can cause their own set of side effects (immune-related adverse events), they generally avoid the systemic toxicity of conventional chemotherapy, leading to a significantly improved quality of life for the patient.
The Ethical and Accessibility Imperative
While the scientific promise of Precision Oncology is immense, its implementation presents real-world challenges. The high cost of comprehensive genomic sequencing and advanced therapies like CAR T-cell therapy raises concerns about equitable access. Furthermore, the sheer volume and complexity of the genetic data require highly specialized clinical and computational expertise, creating a need for increased genomic literacy among healthcare providers worldwide. Addressing these issues is vital to ensure that the advancements in Precision Oncology benefit all patients, regardless of geographic or socioeconomic factors.
Ultimately, the marriage of genomic sequencing and immunotherapy is not just an incremental improvement; it is a fundamental shift in the philosophy of cancer care. By embracing the uniqueness of each patient’s disease through tumor profiling and employing targeted strategies like personalized cancer vaccines and CAR T-cell therapy, the medical community is delivering on the promise of Precision Oncology—less toxicity, higher efficacy, and a brighter future for those facing a cancer diagnosis.