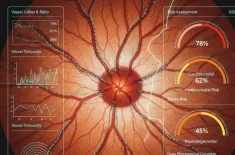
How Multi-omics—integrating genomics, proteomics, and metabolomics—is revolutionizing precision medicine to create a predictive health profile for holistic health and proactive wellness.
For decades, the foundation of medical understanding and health advice rested on single measurements: a cholesterol reading, a blood glucose level, or the presence of a specific gene mutation. While these single-point data provided invaluable insights, they often failed to capture the complexity and dynamism of human biology. Wellness, especially, is not a static state but a continuous biological process influenced by genetics, environment, lifestyle, and time.
The 21st century has ushered in a revolution—a paradigm shift known as Multi-omics. This integrated approach moves beyond isolated data points, combining vast data sets from different biological layers to construct a truly comprehensive and dynamic picture of an individual's holistic health. The goal of this field is to leverage massive computational power and advanced measurement techniques to move precision medicine from a concept primarily focused on disease treatment into the realm of proactive wellness and longevity.
Defining the "Omics" Landscape
Multi-omics is the synchronized study of different 'omic' data sets within an organism. Each 'omic' discipline provides a unique, yet interconnected, view of the biological system. The three foundational pillars driving the current wellness revolution are:
1. Genomics: The Blueprint of Life
Genomics is the study of an organism's entire DNA sequence. It is the fundamental blueprint, providing information about inherent predispositions, susceptibilities to certain conditions, and how an individual is likely to respond to various environmental factors, diets, and medications. In the context of wellness, genomics helps in understanding inherited risk factors and metabolic tendencies, such as the efficiency of certain detoxification pathways or nutrient absorption. Analyzing single nucleotide polymorphisms (SNPs) and structural variations provides the underlying context for an individual's biological operations.
2. Proteomics: The Functional Workforce
Proteomics is the large-scale study of proteins, which are the functional workhorses encoded by the genome. Unlike the relatively static nature of DNA, the proteome is highly dynamic, changing rapidly in response to internal and external stimuli, including stress, exercise, and diet. A protein's abundance, structure, and modifications dictate the actual processes occurring in a cell at any given time. Proteomics offers a current snapshot of biological activity, including immune status, inflammation levels, and cellular stress, which are crucial indicators of holistic health and disease progression.
3. Metabolomics: The End-Product Readout
Metabolomics involves the systematic study of small molecules, known as metabolites, found within cells, tissues, or biofluids. Metabolites—such as sugars, amino acids, lipids, and organic acids—are the end products of cellular processes. As such, the metabolomics profile is the most proximal reflection of the biological reality, directly influenced by gene expression, protein activity, and immediate lifestyle factors (e.g., the food just eaten, the stress of the last hour, the intensity of a workout). This 'real-time' data layer provides critical information for personalized nutrition and lifestyle interventions.
The Synergy of Multi-Omics: Creating the Predictive Health Profile
The true power of Multi-omics is realized through biomarker integration. Simply possessing data from genomics, proteomics, and metabolomics is not enough; the data must be computationally linked to reveal complex biological narratives.
Deeper Insights Through Integration
When these 'omic' layers are analyzed together, they answer more sophisticated questions:
-
Genomics (Potential) Proteomics (Function) Metabolomics (Current State): Does a genetic predisposition for poor vitamin D metabolism (genomics) translate into low active vitamin D protein levels (proteomics), and is the patient currently exhibiting low calcium metabolites (metabolomics)? This integrated approach confirms a functional deficiency stemming from a genetic risk, leading to a high-certainty, actionable recommendation.
-
Systems Biology Approach: Multi-omics embodies a systems biology approach, viewing the human body not as a collection of isolated organs but as an integrated network. By modeling the interactions between genes, proteins, and metabolites, researchers can identify specific molecular mechanisms underlying health states or the early stages of chronic conditions.
The Predictive Health Profile (PHP)
The ultimate goal of this biomarker integration is the creation of a predictive health profile (PHP). This is more than just a risk score; it is a personalized, actionable map that forecasts potential health trajectories.
The predictive health profile utilizes advanced bioinformatics and machine learning to:
-
Quantify Risk: Identify specific, quantifiable risks for conditions like Type 2 diabetes, cardiovascular disease, or neurodegeneration years before traditional diagnostics would flag a problem.
-
Model Interventions: Simulate how specific diet changes, exercise regimes, or nutraceutical interventions are likely to alter the metabolomics and proteomics profiles, effectively modeling the outcome of a personalized wellness plan.
-
Monitor Longitudinal Changes: Provide a dynamic dashboard of health biomarkers that track an individual's response to interventions over time, moving from a static baseline to a continuous state of health monitoring.
Application of Multi-Omics in Proactive Wellness and Longevity
The application of Multi-omics is revolutionizing the wellness and anti-aging medicine space, offering highly tailored, data-driven strategies for optimal health.
Personalized Nutrition and Dietary Interventions
Traditional nutrition advice is often generic. Multi-omics enables true personalized nutrition. By analyzing how an individual's genomics predisposes them to utilize certain fats or carbohydrates, how their proteomics reflects food sensitivities (e.g., inflammatory markers), and how their metabolomics changes in response to specific meals, clinicians can design dietary guidelines with unprecedented accuracy. This leads to recommendations that improve gut health, stabilize blood sugar, and reduce chronic, low-grade inflammation—key drivers of age-related disease.
Stress Resilience and Cognitive Function
Stress impacts the entire biological system, quickly altering the proteome and metabolome (e.g., cortisol and related steroid metabolites). Multi-omics allows for the objective measurement of physiological stress load and allostatic load. Monitoring changes in specific stress-related biomarkers from the proteomics and metabolomics layers enables the creation of personalized stress management and mindfulness protocols aimed at restoring homeostasis and preserving cognitive function and brain health.
Exercise Response and Recovery Optimization
The response to physical exercise is highly individualized. An individual's genomics can hint at whether they are better suited for endurance or power activities. Their proteomics and metabolomics can then track muscular damage, recovery rates (e.g., creatine kinase and lactic acid clearance), and anabolic status. This information is critical for performance optimization and preventing overtraining, helping individuals train smarter based on their unique biological signature.
Challenges and the Future of Multi-Omics for Wellness
Despite its exponential growth, the field of Multi-omics faces critical challenges that must be addressed for its widespread adoption in preventative health.
Data Complexity and Bioinformatics
The sheer volume and complexity of the data generated—terabytes of raw sequence data, thousands of quantified proteins, and hundreds of identified metabolites—demand sophisticated bioinformatics pipelines and powerful data interpretation tools. The key challenge lies in translating complex molecular signatures into simple, clinically actionable recommendations that can be understood and implemented by both practitioners and the public. Standardization of sample collection, measurement, and data processing across laboratories is also essential for data reproducibility.
Cost and Accessibility
Currently, comprehensive Multi-omics testing can be costly, limiting access and hindering the democratization of precision wellness. As sequencing and mass spectrometry technologies become faster and cheaper, similar to the trajectory of genomics, the cost is expected to drop, making a predictive health profile accessible to a broader population seeking health optimization.
Ethical and Regulatory Oversight
As Multi-omics provides increasingly deep and predictive health information, questions around data privacy, ownership, and the ethical use of such potent information must be addressed. Robust data security and clear regulatory guidelines are necessary to maintain public trust and ensure responsible application of these powerful tools in personalized healthcare.
Conclusion: The Era of Personalized and Predictive Wellness
The rise of Multi-omics marks a fundamental inflection point in our understanding of holistic health. By transcending the limitations of single-marker analysis and creating a sophisticated network of information using genomics, proteomics, and metabolomics, we can generate an unprecedented predictive health profile.
This integrated approach is the future of precision medicine—a future where healthcare is not about waiting for a disease to manifest but about dynamically maintaining optimal health and extending healthspan. Multi-omics is not just a scientific curiosity; it is the practical, data-driven engine empowering individuals and practitioners to make highly personalized, proactive decisions for lifelong wellness and resilience. The journey to deep phenotyping for all is well underway, promising a new era of proactive, tailored, and truly personalized wellness.