Learn how sleep deprivation fuels a cortisol spike and drives glucose variability.
The Unseen Link Between Poor Sleep Quality and Metabolic Dysfunction
It’s a common scenario: you toss and turn all night, running on fumes the next morning. You might expect to feel sluggish, groggy, or irritable, but what you might not realize is that the chaos of a sleepless night doesn't just reside in your mind—it's actively reorganizing your blood sugar levels, setting the stage for morning hyperglycemia and a day of glucose variability. This insidious link between poor sleep quality and metabolic disruption is a critical area of health that demands attention, especially for the millions managing or at risk of type 2 diabetes.
Mounting scientific evidence confirms that skimping on or fragmenting your sleep has profound and immediate negative effects on how your body processes sugar. A single night of significant sleep deprivation can reduce your body’s sensitivity to insulin by as much as 25% to 30% in otherwise healthy individuals. When this becomes a chronic pattern, it fundamentally undermines the delicate balance of glucose homeostasis, paving the way for long-term health complications.
The Direct Link: Stress Hormones and Insulin Resistance
The mechanism connecting poor sleep and elevated blood sugar the following day is primarily mediated by hormonal and nervous system responses, with stress hormones playing a starring role.
The Role of Stress Hormones (Cortisol)
The immediate aftermath of insufficient sleep triggers a physiological stress state. In response to what the body perceives as an emergency or disruption, the adrenal glands ramp up production of cortisol, the body’s primary stress hormone.
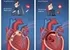
[Image of the Hypothalamic-Pituitary-Adrenal (HPA) axis and its effect on cortisol and blood sugar]
Cortisol follows a natural **circadian rhythm**, typically peaking in the morning to help you wake up and naturally subsiding in the evening to allow for sleep. However, a lack of sleep, fragmented sleep, or chronic stress elevates this already present **cortisol spike**, throwing the body's glucose regulation out of sync.
The function of cortisol is essentially to provide the body with energy to cope with stress—the historical "fight-or-flight" response. To do this, cortisol signals the liver to increase **gluconeogenesis**—the production of "new" glucose from non-carbohydrate sources like amino acids. It floods the bloodstream with sugar, preparing the muscles for action.
At the same time, cortisol actively **impedes the action of insulin**. Insulin is the key hormone that unlocks cells, allowing glucose to move from the blood into the cells for energy or storage. When cortisol is high, it causes cells (especially muscle and fat cells) to become temporarily **insulin resistant**, meaning they ignore insulin’s signal.
The result is a double-whammy:
- Increased Glucose Production: The liver dumps more sugar into the blood due to the heightened cortisol signal.
- Decreased Glucose Clearance: Cells refuse to take up the sugar because they are insulin resistant.
This leaves an excess of glucose circulating in the blood, leading directly to **morning hyperglycemia** (high blood sugar). Over time, repeated episodes of sleep-induced, cortisol-driven insulin resistance can lead to **chronic insulin resistance** and, ultimately, the development of type 2 diabetes.
The Dawn Phenomenon and Sleep's Disruption
While related to the morning rise in blood sugar, the natural **dawn phenomenon** is exacerbated by poor sleep.
What is the Dawn Phenomenon?
The **dawn phenomenon** is a natural, cyclical process that occurs in all people, but is often more pronounced and problematic for individuals with diabetes. Between approximately 3:00 a.m. and 8:00 a.m., the body naturally releases a cocktail of hormones—including growth hormone, glucagon, and, most importantly, **cortisol**—to prepare the body for waking. These hormones instruct the liver to release glucose stores to supply energy for the coming day. In a healthy person, the pancreas responds by producing enough insulin to counteract this release, keeping blood sugar stable.
Sleep Deprivation and Exaggeration
In a state of **sleep deprivation** or poor **sleep quality**, the exaggerated **cortisol spike** amplifies this natural process. The stress on the body from lack of restorative rest means the counter-regulatory hormones are released in greater quantities.
Furthermore, a night of poor sleep is often indicative of fragmented or non-restorative deep sleep (Slow-Wave Sleep), which has been shown to be crucial for maintaining good insulin sensitivity. By suppressing this essential phase of sleep, the body's ability to respond to insulin the next morning is already impaired. The combination of an aggressively high surge of blood-sugar-raising hormones and decreased insulin sensitivity leads to a significantly higher and more sustained level of **morning hyperglycemia** compared to a well-rested state.
The Rollercoaster: Glucose Variability
The impact of poor sleep extends beyond a simple elevated fasting glucose reading; it creates a state of metabolic instability known as increased **glucose variability**.
Defining Glucose Variability
**Glucose variability (GV)** refers to the magnitude and frequency of fluctuations in blood sugar levels throughout the day and night. High GV—a "rollercoaster" of peaks and valleys—is recognized as an independent risk factor for diabetes complications, potentially causing more damage to blood vessels than consistently high, but stable, blood sugar.
How Sleep Deprivation Fuels GV
The hormonal and metabolic disruption caused by sleep loss directly increases this variability:
- **Morning Spike:** The hyper-responsive **cortisol spike** and reduced insulin sensitivity lead to the immediate, sharp spike of **morning hyperglycemia**.
- **Daytime Instability:** Starting the day with high sugar levels makes it much harder to stabilize them, leading to exaggerated responses to meals and greater swings between high and low glucose throughout the day.
- **Cravings and Hormones:** Sleep deprivation also disrupts the appetite-regulating hormones leptin (satiety) and ghrelin (hunger), increasing cravings for high-carbohydrate, energy-dense foods. Consuming these foods while in an insulin-resistant state further amplifies blood sugar spikes.
Essentially, poor sleep sabotages the body's baseline ability to maintain stable sugar levels, trapping the individual in a perpetual cycle of highs and lows.
Reclaiming Control: The Power of Sleep Hygiene
Given the potent and immediate impact of sleep on glucose metabolism, improving **sleep hygiene** is arguably as vital for metabolic health as diet and exercise. **Sleep hygiene** refers to a set of practices and habits necessary to promote sound **sleep quality** and quantity.
Strategies for Metabolic-Focused Sleep
| Sleep Hygiene Focus | Action for Blood Sugar Control |
|---|---|
| Consistency | Maintain a strict, regular sleep schedule (bedtime and wake-up time), even on weekends. This stabilizes your **circadian rhythm** and helps regulate the natural **cortisol spike**. |
| The Sleep Environment | Ensure your bedroom is dark, cool, and quiet. Darkness is essential for melatonin production, the hormone that promotes sleep and helps quiet the brain’s stress response. |
| Pre-Bed Routine | Implement a relaxing wind-down routine 30-60 minutes before bed. This should exclude blue light from electronics, which can suppress melatonin and increase alertness. |
| Timing of Stimulants | Avoid caffeine and heavy meals close to bedtime. Caffeine can disrupt sleep architecture, and a large meal (especially high-carb) can cause glucose spikes that interfere with restful sleep and worsen **morning hyperglycemia**. |
| Nighttime Monitoring | For individuals with diabetes, regular nighttime blood glucose checks or Continuous Glucose Monitoring (CGM) can identify nocturnal highs or lows that are fragmenting sleep. Addressing these with your healthcare provider is key. |
By prioritizing adequate **sleep quality** (7-9 hours for most adults) and focusing on established **sleep hygiene** practices, individuals can directly mitigate the cortisol-driven insulin resistance and reduce harmful **glucose variability**, bringing much-needed stability to their metabolic system.